Open Access | Peer-reviewed | Review Article
Chee Kong Yap*
Department of Biology, Faculty of Science, Universiti Putra Malaysia,43400 UPM, Serdang, Selangor, Malaysia.
Published: April 22, 2020 DOI: 10.5281/zenodo.3761652
Abstract
A novel corona virus (SARS-CoV-2) outbreak is claiming thousands of lives worldwide. As of April 10, 2020, the number of laboratory-confirmed cases of SARS-CoV-2 infected has reached over 1.7 million (1,725,126) with over 0.1 million (104,878) recorded deaths. This pandemic has ushered an urgent need for identifying drugs which can inhibit the survival of SARS-CoV-2. On one hand, researchers from across globe are searching for various sources of potential antiviral compounds. On the other hand, high diversity, ecological adaptation, defensive system against wide range of viruses makes marine bivalves as a great source of antiviral compounds. Ocean environment has provided a rich diversity of compounds from structural classes including alkaloids, terpenoids, steroids, polysaccharides, and peptides etc., many of which have shown potential activities against bacterial, fungal, parasitic and viral diseases. In this scenario, potential antiviral compounds from marine bivalves, worth evaluating against SARS-CoV-2 have been briefly summarized in this present review.
Keywords: Antiviral compounds; marine bivalves; antiviral potency; SARS-CoV-2; novel corona virus.
| Citation: Chee Kong Yap (2020) Antiviral compounds from marine bivalves for evaluation against SARS-CoV-2, Journal of PeerScientist 2(2): e1000015. |
| Received: April 01, 2020; Accepted April 21, 2020; Published April 22, 2020. |
| Copyright:© 2020 Chee Kong Yap. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. |
| Data Availability: All relevant data are within the paper and its Supporting Information files. |
| Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. |
| Competing interests: The authors have declared that no competing interests exist. |
| * E-mail: yapckong@hotmail.com | Phone: + 603-9769 6616. |
Introduction
A novel corona virus (nCoV) outbreak in late 2019 at Wuhan, China is claiming thousands of lives across globe leaving humans in a predicament situation of novel corona virus disease (COVID-19) pandemic [1]. As of April 10, 2020, the number of laboratory-confirmed cases of SARS-CoV-2 infected has reached over 1.7 million (1,725,126) with over 0.1 million (104,878) recorded deaths [2]. The incremental numbers for both total cases and total deaths signifies that the coronavirus is infecting humans exponentially. Present identified novel corona virus belongs to same family of recent Severe Acute Respiratory Syndrome (SARS) and Middle East Respiratory Syndrome (MERS) outbreaks [3-4]. Zhang et al. 2002 showed that the outbreaks of the Severe Acute Respiratory Syndrome (SARS) in many regions of Asia were well described by the logistic model, and the control measures implemented by governments are effective [5]. Christian et al. 2014 reported that the number of patients diagnosed positive with Middle East Respiratory Syndrome (MERS) are with known travel history between cities in King Fahd Hospital and all other hospitals, between March 26 until April 28, 2014 [6]. The above two studies shows the peak of coronavirus COVID-19 shall be reaching a plateau and this level-off pattern would indicate the fall of the pandemic overall cases. Presumably, any viral outbreak will eventually become less contagious and harmful, and less of the public health concern. Only the discovery or repurposing of potential antiviral drugs (ADs) can be a good answer if not the best to cure and save the infected patients’ lives at present, and to control this viral pandemic COVID-19 spread.
Figure 01: 2D structures of murexine, tyrindoleninone; 6-bromoisatin, 6,6’-dibromoindirubin and 6,6’-dibromoindigo compounds.
Oceans hold exclusive supplies that can offer a wide spectrum of natural products, principally from invertebrates such as bivalves. As infectious diseases change and grow endurance to existing pharmaceuticals, the marine environment can provide an innovative protection against bacterial and viral diseases [7]. Limitations in antiviral treatments and the occurrence of novel pathogenic viruses have added to a mounting requirement for novel and applicable chemotherapeutic agents to cure viral diseases. Researches on the chemical structures and Antiviral Compounds (ACs) [8] of uncommon metabolic products of aquatic life have proven that marine organisms can provide excellent outlooks in the hunt for ADs [9]. Owing to the fact that bivalves are in scarcity of a distinct adaptive immune system, they must utilize their inborn immune system as a protection against viral infections [10-11]. Besides the corporal obstacles of skin, shell, and epithelium, the bivalves’ inborn immunity is also amplified by the occurrence of numerous antimicrobial chemical agents [12]. Biological diversity and ecological adaptations of bivalves hint at a potential source of novel ACs for future AD discovery. In addition, ACs are a vital part of bivalves’ defences against viruses with varied mechanisms of action against a wide array of viruses, including human pathogens [13]. In this scenario, this review aims at presenting potential antiviral compounds from marine bivalves, worth evaluating against SARS-CoV-2.
 Figure 02: 2D structure of mytilin compound from Mytilus galloprovincialis.
Figure 02: 2D structure of mytilin compound from Mytilus galloprovincialis.
Figure 03: 2D structure of Defensin compound from Mytilus galloprovinciali.s.
Tincu, J. A., and Taylor, S. W 2004 observed antimicrobial agents from marine bivalves can counteract the invasions of pathogenic microbes effectively [14]. Olicard, C et.al 2005 [15] and Carriel‐Gomes et.al. 2006 [16] demonstrated antiviral activity in hemolymph from oysters, Crassostrea gigas and C. rhizophorae. Bilal Muhammad Khan and Yang Liu, 2019 summarized that secondary metabolites isolated from bivalves including mussel Mytilus galloprovincialis, mussel Crenomytilus grayanus, clam Mercenaria mercenaria, clam Ruditapes philippinarum, cockle Cerastoderma edule, clam Myaarenaria, oyster C. gigas, oyster C. virginica, and oyster Ostrea edulis have been investigated as having AP against many human related viruses [17]. Still, there are about 100,000 species of mollusks that remain unverified for potential AP [13]. Kirsten Benkendorff 2009 reported murexine, tyrindoleninone; 6-bromoisatin, 6,6’-dibromoindirubin and 6,6’-dibromoindigo compounds as some of the important drug like compounds from mollusks (figure 01) [18]. In recent review by Odeleye et al. 2019, summarized main health benefits of molluscan extracts include anti-cancer, antioxidant, and anti-infectious disease activities [19]. Chatterji et al. 2002 reported that the extracts from economically important estuarine clam Meretrix casta, mussel P. viridis, mud clam Polymesoda erosa, oyster C. madrasensis, giant oyster C. gryphoides, and black clam Villorita cyprinoide were found to reduce the infection of influenza virus type-A and B to a great extent [20].
Figure 04: 2D structures of Tachyplesin I & II compounds from Tachypleus tridentatus and Limulus polyphemus.
A comprehensive review by Dang et al. 2015 summarized most characterized extracts from bivalve species with in vitro antiviral activity. Mytilin (figure 02) and Defensin (figure 03) from Mytilus galloprovincialis showed direct inhibition of viral attachment and transcription process. Defensins are cysteine rich 18-45 amino acid length peptides produced by innate immune system and found highly active against a variety of bacteria, fungi, enveloped and non-enveloped viruses [13]. On the other hand, Tachyplesin I & II (figure 04) are another set of isopeptides isolated from hemocytes of horseshoe crab (Tachypleus tridentatus and Limulus polyphemus) were shown to directly inactivating several viruses including Vesicular stomatitis virus (VSV) and Influenza (H1N1) virus [21].
Figure 05: 2D structures of Dolastatin 10 & 15, Kahalaide F and Keenamide A compounds.
Premalata Pati et.al, 2014 summarized unique secondary metabolites with demonstrated anti-microbial activies. Dolastatin 10 & 15, Kahalaide F, Keenamide A (figure 05), Turbostatin 1, Spisulosine-ES-285, Ulapualide-A, and Ziconotide (figure 06) are some of the biologically active extracted, identified and isolated from marine molluscs [22]. Figure 06: 2D structures of Turbostatin 1, Spisulosine-ES-285, Ulapualide-A, and Ziconotide compounds.
Figure 06: 2D structures of Turbostatin 1, Spisulosine-ES-285, Ulapualide-A, and Ziconotide compounds.
Maria Jose Abad Martinez et.al, 2008 reviewed literature and summarized compounds from marine sources which are active against Human Immunodeficiency Virus (HIV) are Avarol, avarone, lamellarins, dragmacidin F, toxiusol; Herpes Simplex Virus (HSV) are 4a-acetyldictyodial, pseudopterosins, helioporins, 8-hydroxymanzamine A, halovirs; Cytomegalovirus (CMV) are Sulfolipids, plastoquinones, pseudopterosin P and Influenza (H1N1) virus are Nostoflan and strongylin A; Human T- cell leukemia virus , type 1 (HTLV-1) are Fistularin 3, 11-ketofistularin 3, kelletinin A; murine coronavirus is Halitunal; Hepatitis B (HBV) are neofolitispates, pentacyclic guanidine alkaloids, Alpha-galactosylceramide and clavulone against Vesicular Stomatitis Virus (VSV) (figure 07) [7].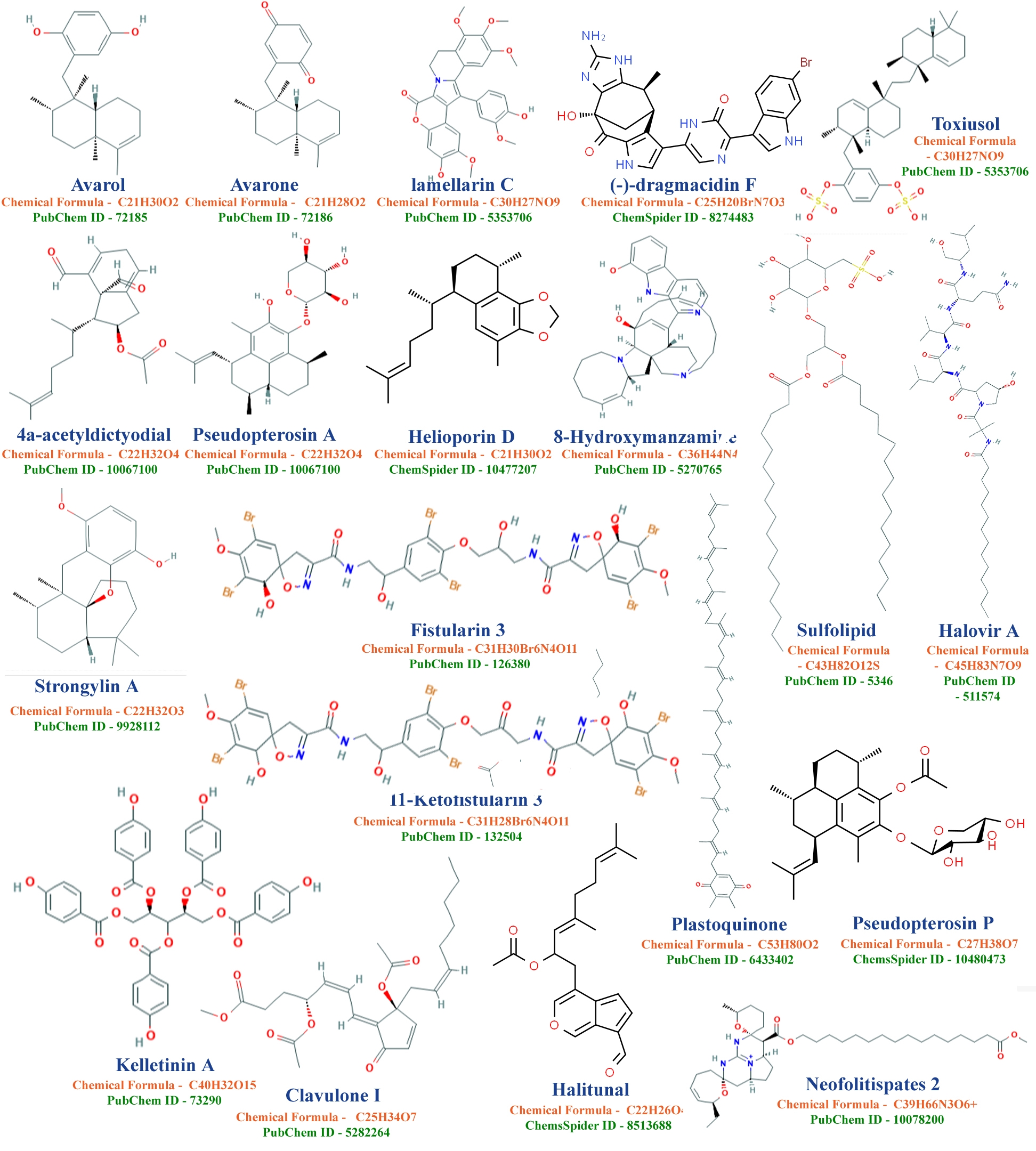
Figure 07: 2D structures of Dolastatin 10 & 15, Kahalaide F, Keenamide A, Turbostatin 1, Spisulosine-ES-285, Ulapualide-A, and Ziconotide compounds.
Muhammed Zafar Iqbal and Khan 2016 reported a novel DNAse like compound that can inhibit viral propagation from the green-lipped mussel Perna viridis. They explored DNAse like bioactivity for natural non-proteinacious compound(s) extracted from P. viridis. These results indicated the prospect of a source of potential AD against DNA Group I viruses. Muhammed Zafar Iqbal and Khan speculated that the marine mussels have evolved some mechanisms against viral infections which need further studies [22].
Conclusion
In conclusion, all the above literature indicates that various marine sources, especially marine bivalves holds rich diversity of compounds from structural classes including alkaloids, terpenoids, steroids, polysaccharides, and peptides etc., with potential antiviral potency which can be used for further studies towards converting them into antiviral drugs. Owing to the fact that bivalves are totally depended on their inborn immune system to protect themselves against viral infections, they are bound to develop a wide range of compounds which are hard to find elsewhere. Taking these compounds as start point, potential target specific drug like compounds can be designed to tackle viral infections, including present SARS-CoV-2 infections.
References
- Zhu, Na, et al. “A novel coronavirus from patients with pneumonia in China, 2019.” New England Journal of Medicine(2020).
- Worldometer https://www.worldometers.info/coronavirus/ (Accessed on April 11, 2020).
- Ruan, YiJun, et al. “Comparative full-length genome sequence analysis of 14 SARS coronavirus isolates and common mutations associated with putative origins of infection.” The Lancet9371 (2003): 1779-1785.
- WHO MERS-CoV Research Group. “State of knowledge and data gaps of Middle East respiratory syndrome coronavirus (MERS-CoV) in humans.” PLoS currents5 (2013). doi: 10.1371/currents. outbreaks.0bf719e352e7478f8ad85fa30127ddb8
- Zhang, Z., Sheng, C., Ma, Z. and Li, D“The outbreak pattern of the SARS cases in Asia.” Chinese Sci. Bull., 49(17) (2002):1819-1823.
- Christian, D., Muth, Doreen, M., Victor, C., Hussain, Raheela, H., Malaki, M., Waleed, O., Olfert, L., Abdullah, A., Isabella, E., Ali, S., Jaffar, Al-T., Ali, A., Alimuddin, Z., Andrew, R., and Ziad, M. “An observational, laboratory-based study of outbreaks of middle east respiratory syndrome coronavirus in Jeddah and Riyadh, Kingdom of Saudi Arabia.” Infect. Diseases. (2014). DOI: 10.1093/cid/ciu812.
- Abad Martinez, M.J., Bedoya Del Olmo, L.M., and Bermejo Benito, P. “Natural marine antiviral products.” Nat. Prod. Chem., 35C (2008): 101-134.
- Basha, Syed Hussain. “Corona virus drugs–a brief overview of past, present and future.” Journal of PeerScientist 2 (2020): e1000013.
- Che, C.‐ “Marine products as a source of antiviral drug leads.” Drug Develop. Res., 23(3) (1991): 201-218.
- Hooper, C., Day, R., Slocombe, R., Handlinger, J., and Benkendorff, K. “Stress and immune responses in abalone: Limitations in current knowledge and investigative methods based on other models.” Fish Shellfish Immunol., 224 (2007): 363–379.
- Tiscar, P., and Mosca, F. “Defense mechanisms in farmed marine molluscs.” Res. Comm., 281 (2004): 57–62.
- Bachere, E., Mialhe, E., Noel, D., Boulo, V., Morvan, A., and Rodriguez, J. “Knowledge and research prospects in marine mollusc and crustacean immunology.” Aquaculture, 1321 (1995): 17–32.
- Dang, V. T., Benkendorff, K., Green, T., and Speck, P. “Marine snails and slugs: A great place to look for antiviral drugs.” Virol., 89 (2015): 8114–8118.
- Tincu, J. A., and Taylor, S. W. Antimicrobial peptides from marine invertebrates. Antimicrobial Agents Chemotherapy, 4810 (2004): 3645-3654.
- Olicard, C., Renault, T., Torhy, C., Benmansour, A., and Bourgougnon, N. “Putative antiviral activity in hemolymph from adult Pacific oysters, Crassostrea gigas.” Antiviral Res., 662–3 (2005): 147-152.
- Carriel‐Gomes, M. C., Kratz, J. M., Müller, V. D., Barardi, C. R., and Simões, C. M. “Evaluation of antiviral activity in hemolymph from oysters Crassostrea rhizophorae and Crassostrea gigas.” Liv. Resour., 192 (2006):189–193.
- Khan, B. M., and Liu, Y. “Marine mollusks: Food with benefits.” Rev. Food Sci. Food Saf., 18 (2019):548-564.
- Benkendorff, K. “Molluscan biological and chemical diversity: Secondary metabolites and medicinal resources produced by marine molluscs.” Rev., 85(4) (2010): 757–775.
- Odeleye, T., White, W.L., and Lu, J. “Extraction techniques and potential health benefits of bioactive compounds from marine molluscs: A review.” Food Funct., 105 (2019): 2278-2289.
- Chatterji, A., Ansari, Z.A., Ingole, B.S., Bichurina, M.A., Sovetova, M., and Boikov, Y.A. “Indian marine bivalves: Potential source of antiviral drugs.” Sci., 8210 (2002): 1279-1282.
- Murakami, Tsukasa, et al. "Direct virus inactivation of tachyplesin I and its isopeptides from horseshoe crab hemocytes." Chemotherapy5 (1991): 327-334.
- Pati, P., Sahu, B.K., and Panigrahy, R.C. “Marine molluscs as a potential drug cabinet: An overview.” Indian J. Geo-Mar. Sci., 44(7) (2015): 961-970.
- Muhammed Zafar Iqbal, A.N., and Khan, M.S. “A novel DNAse like compound that inhibits virus propagation from Asian green mussel, Perna viridis” Indian J. Exp. Biol., 54 (2016): 816-821.